A message from Kings Liver Pharmacy: Update to the immunosuppression protocol for liver transplant patients

From September 12th 2022, there will be a change to the immunosuppression protocol used for liver transplant patients. This will not affect existing transplant patients who are well established on their immunosuppression but will mean that newly transplanted patients will be on a different brand of tacrolimus to existing patients. We are not planning to switch stable patients to the new formulation until a thorough service evaluation has been completed.
We will be switching our first-line brand of tacrolimus from Advagraf® to Envarsus®, which is also a once-daily formulation of tacrolimus.
We have recently carried out a service evaluation of Envarsus® and have found that patients who were suffering from side effects of tacrolimus (mainly tremor and headaches) had a significant improvement in symptoms following the switch. We also found that patients needed a significantly lower daily dose of tacrolimus in order to maintain the target tacrolimus levels.
Patients who have had surgery to remove any part of their small intestines will not be prescribed Advagraf® or Envarsus® but will be prescribed the twice-daily formulation of tacrolimus, Prograf®.
We will continue to evaluate this new protocol following its implementation to ensure that there are no unexpected issues as a result of the switch in tacrolimus brands.
Recent Posts
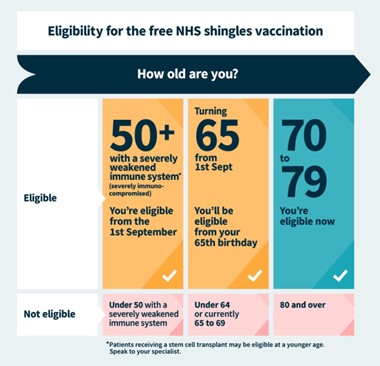
- New Shingles Vaccine and Immunisation Programme
- A message from Kings Liver Pharmacy: Update to the immunosuppression protocol for liver transplant patients
- Measuring Immunosuppressive Drugs – presentation by Phil Morgan at Listen Group Zoom meeting July 2022
- Psychological Wellbeing – Liver Transplant
- Possible Recording of Group Meetings